Nuclear cardiac imaging techniques such as SPECT and PET are excellently suited for assessing myocardial blood flow disorders. Therefore, a radioactive substance (i.e. the tracer) is injected intravenously at rest and during physically or pharmacologically induced stress conditions. Image acquisition of the regional tracer uptake allows for depiction of ischemia or scar tissue within the left ventricular myocardium. Furthermore, exact localization and assessment of the extent of ischemic myocardium allows determining an optimal individualized treatment strategy for each patient (e.g. coronary artery bypass graft operation, percutaneous coronary intervention, or optimal medical therapy).
SPECT offers evaluation of relative myocardial perfusion uptake within the myocardium. This approach, however, demands that at least one coronary territory must be perfused normally in order to serve as a reference territory for detection of perfusion defects. By contrast, PET myocardial perfusion imaging enables quantitative evaluation of the myocardial blood-flow (in ml x min-1 x g-1 myocardium), leading to a higher diagnostic accuracy and additionally allowing for assessment of microcirculatory system for example if small vessel disease is suspected. Of note, the radiation dose exposure associated with a myocardial perfusion PET study is only 3-5 mSv, which is substantially lower than the radiation dose exposure arising from a myocardial perfusion SPECT study which currently adds up to 8-10 mSv.
By contrast, CCTA does not currently offer assessment of myocardial perfusion but rather delivers excellent anatomical and morphological information particularly of the coronary arteries through exact depiction of coronary calcifications and stenoses (Fig 1). Spatial resolution has seen major improvements and radiation dose exposure has constantly been reduced over the last years, now reaching a mean of 0.5 mSv in daily clinical routine at our institution. The strength of CCTA lies foremost in its excellent negative predictive value, allowing exclusion of coronary artery disease with a certainty of almost 100% if a study is normal.
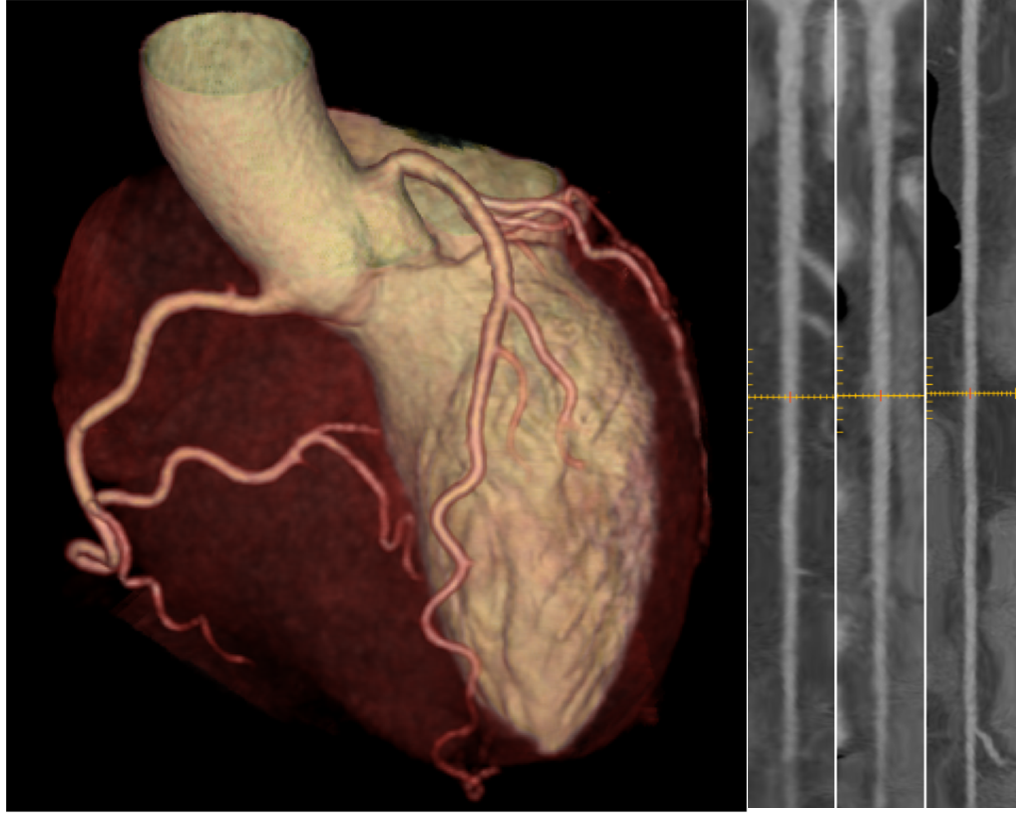
Figure 1. Volume rendering (left panel) gives a general impression on the anatomical course of the coronary arteries for example for excluding coronary anomalies. The coronaries may be depicted in a strechted manner (a so called multiplanar curved reformat; right panels) to better visualize coronary artery lesions. This patient has normal coronary arteries.
Similar to the nuclear cardiac imaging modalities, cMRI allows for characterization of the myocardial perfusion through image acquisition at rest and during pharmacologically induced stress. Instead of radiopharmaceuticals, a contrast agent consisting of gadolinium is applied. The real strength of cMRI, however, lies more in the very detailed depiction of the cardiac anatomy including the cardiac valves and its excellent ability to assess myocardial function.
Each of the abovementioned modalities offers distinct advantages and disadvantages dependent on the clinical question. Thus, in many cases, multimodality imaging or hybrid imaging is a reasonable approach: Combining CCTA with SPECT or PET allows for assessment of the hemodynamic relevance of a coronary lesion detected by CCTA as well as for a clear allocation of an ischemic area to a coronary vessel territory (Fig. 2) enabling a very targeted interventional treatment strategy.
Because of the variety of cardiac imaging modalities at hand, choosing the most appropriate technique for an individual patient may sometimes be difficult. Therefore, please do not hesitate to contact us in case of questions or uncertainties (via direct phone line +41 44 255 15 01).
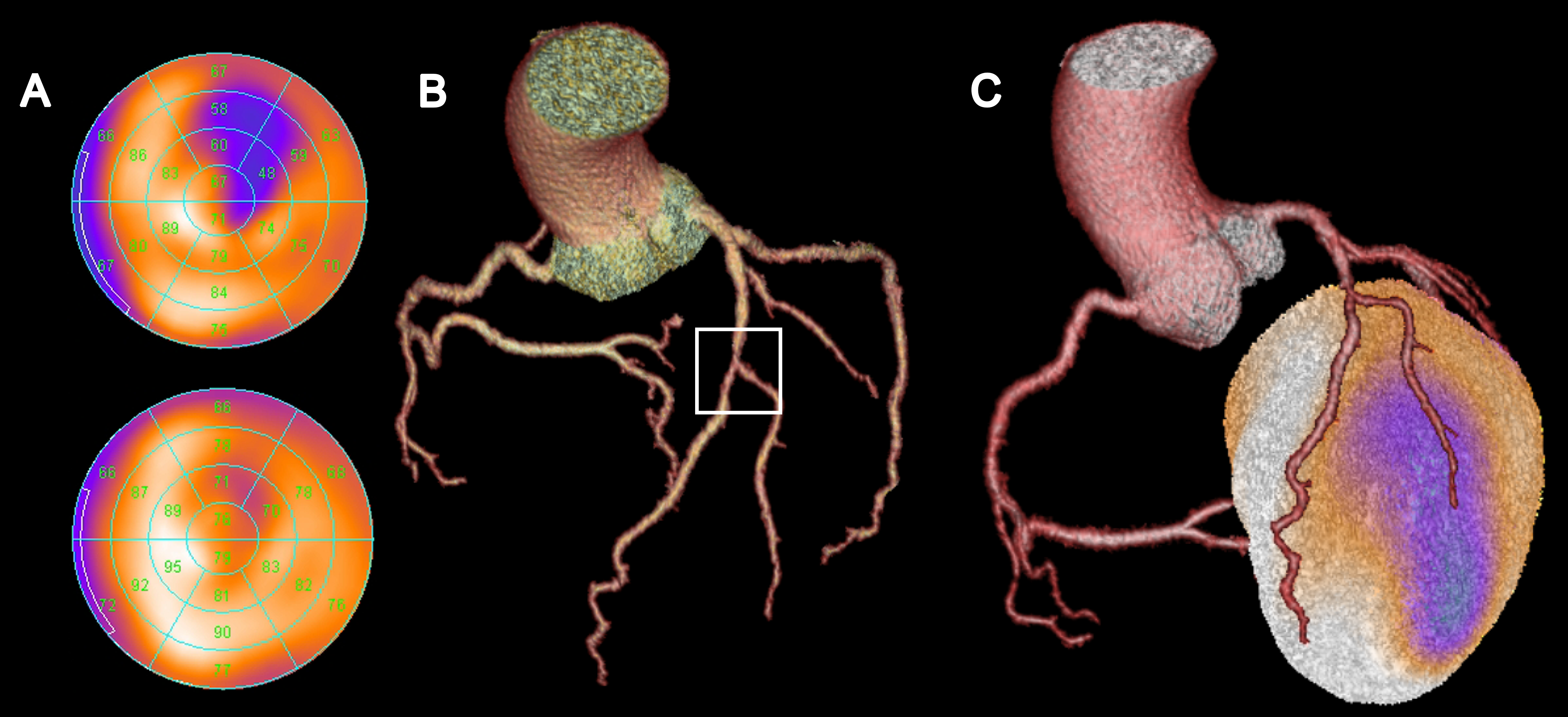
Figure 2. Myocardial perfusion SPECT (A) shows normal perfusion at rest (bottom plot) but a perfusion defect under stress conditions (blue areas, top plot), consistent with a myocardial ischemia in the anterolateral myocardial wall. CCTA (B) depicts a high-grade stenosis in the middle of the left anterior descending artery (LAD) near the bifurcation of the second diagonal branch. Hybrid SPECT/CCTA (C) enables clear association of the ischemic area to the vessel territory of the diagonal branch. By contrast, the vessel territory of the distal LAD does not show any evidence for ischemia and, thus, does not need treatment.



